POLY ALUMINIUM CHLORIDE
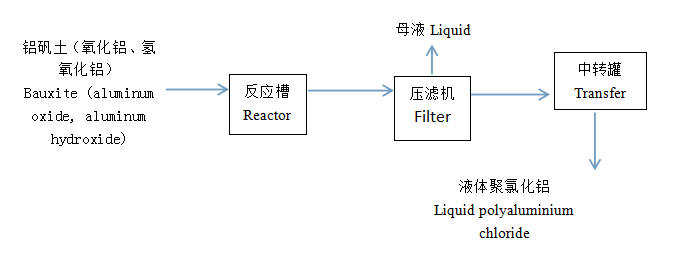
Bauxite (alumina, aluminum hydroxide) is added to the reaction tank, and then hydrochloric acid is added, and then pumped into the reaction tank (polymerization kettle),
Bauxite (alumina, aluminum hydroxide) is added to the reaction tank, and then hydrochloric acid is added, and then pumped into the reaction tank (polymerization kettle),
聚合氯化铝POLY ALUMINIUM CHLORIDE
分子式
Al2O3+6HCl=2AlCl3+3H2O
2Al(OH)3+(6-n)HCl=Al2(OH)nCl6-n+H2O
mAl2(OH)nCl6-n=[Al2(OH)nCl6-n]m
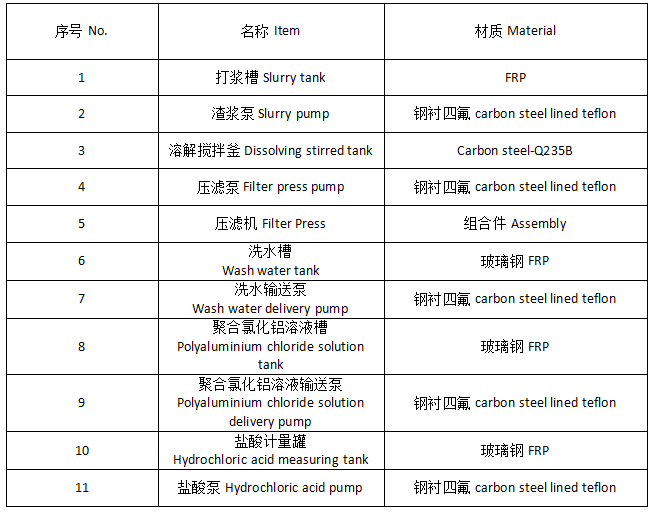
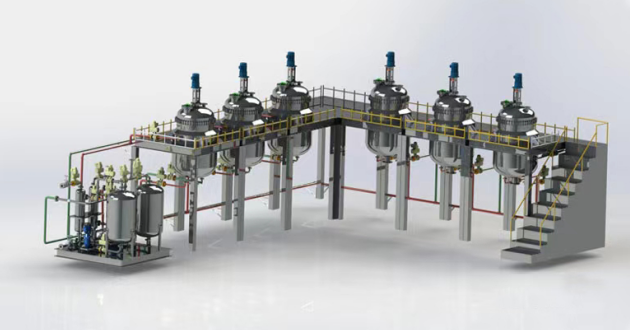
Bauxite (alumina, aluminum hydroxide) is added to the reaction tank, and then hydrochloric acid is added, and then pumped into the reaction tank (polymerization kettle), and calcium aluminate powder is added to adjust the pH value of the slurry to polymerize aluminum ions. Then, the slurry is concentrated according to the aluminum content in the slurry to obtain the finished polyaluminum chloride product, which is pumped into the finished product tank for sale.
Formula
Al2O3+6HCl=2AlCl3+3H2O
2Al(OH)3+(6-n)HCl=Al2(OH)nCl6-n+H2O
mAl2(OH)nCl6-n=[Al2(OH)nCl6-n]m
Add a certain amount of water to the reaction tank, start stirring, and then prepare the bauxite (aluminum oxide, aluminum hydroxide) reaction tank into slurry, add hydrochloric acid to react, and adjust the pH value of the liquid after the reaction is completed to polymerize aluminum ions. Pump it into the filter press to filter and obtain the finished polyaluminum chloride product, which is pumped into the finished product tank for sale.